Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
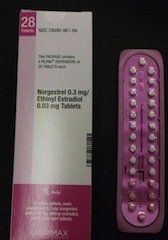
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA


title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-10-12” author: “Sue Heimbach”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-09-22” author: “Charlene Gregory”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-10-11” author: “Phillip Kelly”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-09-10” author: “Terra King”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-10-03” author: “Major West”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-09-08” author: “Emma Hannah”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA



title: “Massive Birth Control Recall Poses Terrifying Risk” ShowToc: true date: “2024-09-19” author: “Geoffrey Tolson”
Yes, today brings what must be positively terrifying news for a lot of women: Pfizer is recalling one million blister packs of birth control pills because a packaging error might mean women who are taking them don’t get the proper dosage and are at a significantly higher risk for an unintended pregnancy.
Whoa! CAN YOU IMAGINE? OK, OK, let’s just calm down here. Key details first: The issue is with specific lots of Lo/Ovral-28 tablets and generic Norgestrel and Ethinyl Estradiol tablets, manufactured by Pfizer and marketed by Akrimax Rx Products under the Akrimax Pharmaceuticals brand. (Click here for the affected lot numbers.) While the blister packets containing the pills are each supposed to have 21 active tablets and 7 inactive sugar tablets, to regulate the taker’s cycle, due to a mix-up, some of the packets have too many active tablets … and some too few! Here’s what Pfizer’s press release about the recall said: Got that? Geez. If this affects you, I’m so sorry. But try not to panic. Take it one step at a time. Just to repeat, lot numbers are listed here. And you can look at photos of the affected brands here. Are you affected by this recall? Image via FDA


